Opinion 
 Creative Commons, CC-BY
Creative Commons, CC-BY
Fibroblast the Non-Professional Immune Cell
*Corresponding author: Titus A Reaves, Department of Regenerative Medicine and Cell Biology, Medical University of SC 173 Ashley Avenue Charleston, SC 29425, USA.
Received:May 20, 2022; Published: June 13, 2022
DOI: 10.34297/AJBSR.2022.16.002247
Introduction
Traditionally, fibroblasts can be defined as mesenchymal cells and are known for producing upwards of 300 unique extracellular matrix (ECM) components and proteins. This includes collagens, proteoglycans, elastin, and cell-binding glycoproteins, each with distinct physical and biochemical properties that must interact to form functional connective tissue [1]. Connective tissue maintains form to the body and organs that provide cohesion and internal support [2,3].
Origin
In terms of germ layers, fibroblasts are derived from mesoderm. However, epithelial mesenchymal transformation is a process where epithelial cells (derived from endoderm) can give rise to fibroblast [4]. Conversely, mesenchymal epithelial transformation is a process where fibroblasts can give rise to epithelial cells [4,5].
Myofibroblast
Fibroblasts are major structural cells that reside in nearly all organs that can differentiate into tissue specific subpopulations during morphogenesis [6]. Also, during morphogenesis, fibroblasts become highly activated, their numbers increase, and as a result activated fibroblasts are defined as myofibroblasts [7,8]. Myofibroblasts express α-smooth muscle actin allowing them to contract tissue during organ injury and/or wound healing. Post development, myofibroblasts rapidly become quiescent, lose contractile activity and return to the nonactivated state [8].
Fibroblasts rapidly return to the myofibroblast phenotype during the inflammatory, proliferative, and maturation phases of wound healing [8]. If there is no resolution to the wound healing, myofibroblasts continue to persist, which result in excessive and dysregulated production of ECM and unwarranted contraction of the affected tissue [9]. These events may lead to fibrosis and a fibrotic phenotype. Fibrosis typically results from chronic inflammation and the affected organ typically do not function effectively and may not function at all [9,10] Myofibroblasts can also arise from exposure of the un-stimulated fibroblast to TGFβ1, insulin-like growth factor 1, interleukin-4, and platelet derived growth factor [11]. Myofibroblast can arise from fibrocytes which are bone marrow derived progenitor cells and circulating progenitor cells also from the bone marrow. Fibrocytes possess properties of both the fibroblasts and the leukocyte (hence the name, [12]). Pericytes can also differentiate into myofibroblasts under inflammatory conditions. Pericytes are contractile cells of mesenchymal origin that are normally present in the walls of both normal and microvessels; they also have a close association with the endothelium [13].
Reported Function
The general overall function of the fibroblasts is to produce ECM and repair damaged tissue [14]. However, there are functional differences in fibroblast depending on the individual organ and the cellular microenvironment [14]. Fibroblast may respond to and in some cases release cytokines and growth factors that can govern both their differentiation and the differentiation of cells in close apposition [14]. In particular, fibroblasts in the heart releases cytokines; are the most abundant cell that interact with the cardiac myocytes during development and during remodeling that follows hypertension, myocardial infarction and/or heart failure [15]. In the kidney, evidence have shown that resident fibroblasts are also actively involved in initiating and promoting inflammation during kidney injury [16]. Such fibroblasts as they age, differentiate into several distinct phenotypic fibroblasts, which includes CXCL13/ CCL19-producing fibroblasts and retinoic acid-producing fibroblasts where in response to injury results in uncontrolled aberrant inflammation and inhibits tissue repair [16]. In the brain, fibroblasts in the periphery of the CNS, play a role in inflammatory signaling, they recruit professional immune cells to site of injury using chemokines and chemoattractant molecules, affect leukocyte trans endothelial migration, and promote immune cell survival through reducing apoptosis [17]. Yet, there are spinal cord, meningeal, chloroid plexus stroma, and perivascular fibroblast with probable slightly different functional responsibilities [17]. New discoveries made by lineage tracing and targeted gene deletion of mesenchymal cell subpopulations, have expanded and reinforced the conclusion that mesenchymal fibroblasts in the lung are crucial for forming and maintaining vascular networks, sensing damage, recruiting inflammatory cells, and remodeling the extracellular matrix of the lung [18].
Expansion of functions non-professional immune cells
Myofibroblast have the capability to function as a nonprofessional immune cell and potentially their respective responsibilities should be expanded. Interestingly, cultured intestinal fibroblasts express toll-like receptors (TLRs)—indicating that they possess the capacity to participate in the identification, control, and possibly the elimination of microbes and their products [19]. In addition, studies show that fibroblasts also express\release myeloperoxidase (MPO). MPO is a heme protease predominantly secreted by polymorpho-nuclear leukocytes (PMN) and functions to destroy pathogenic organisms and mark damaged tissue for removal [20]. They express FPR1 receptor [21]. This is an activation receptor that stimulates leukocytes to upregulate receptors and migrate to the sites where bacterial formylated peptides are present [22]. The goal is to destroy the bacteria [22]. However, A study has shown that in scleroderma, the FPR1 receptor plays a different role. The FPR1 is involved in the differentiation of fibroblasts to a myofibroblasts phenotype [21]. Fibroblasts also express lysozyme, which is also known as muramidase or Nacetylmuramide glycanhydrolase, an antimicrobial enzyme produced at mucosal surface and is part of the innate immune system [23]. Lysozyme is present in human tears, gastric secretions, nasal mucus, and egg white [23]. Lactoferrin is expressed by myofibroblast and is of the transferrin family, a globular glycoprotein that is present at mucosal surfaces in secretory fluids, such as milk, saliva, tears, and nasal secretions [24,25]. Lactoferrin is also present in secondary granules of PMNs [25]. In the intestine, fibroblasts are in a peri cryptal or subepithelial orientation. Interestingly, this location allows the fibroblast to be the last barrier should there be an injury or damage to the epithelial surface, which could allow toxic substances to enter the body. Fibroblast are also scattered throughout the stromal and lamina propria areas of the intestine along with other immune cells [26] (Figure 1). While it a bit hard to determine actual numbers, fibroblasts are a highly abundant cell. Thus, performing as a nonprofessional immune cell is appropriate for such an abundant cell. Can the fibroblast modulate immune system responses? the Kollias lab showed in vivo that intestinal myofibroblasts play a critical role following epithelial injury by sensing the inflammatory milieu generated in the microbial components and activates the Tpl2Cox-2- PGE2 molecular pathway which pro-motes epithelial proliferation [26]. Several labs have shown through invitro experiments, that when myofibroblasts plate and allowed to become confluent on the lower surface of a transwell apparatus, and treated with IL- 6, they will released IL8. Then PMN will migrate from the upper chamber toward the fibroblasts that have release the IL8 (Reaves et al. unpublished observations). The answer is yes, the fibroblast (myofibroblasts) can regulate and propagate inflammation. Further evidence that fibroblast in addition to possessing proteins and receptors that can destroy microorganisms, can regulate immune system responses.
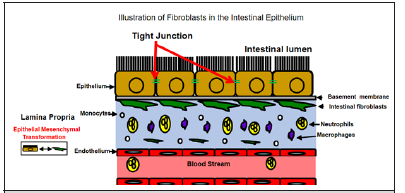
Figure 1: The majority of fibroblasts in the large intestine are localized in a pericryptal orientation. Fibroblast forms a cellular network that separates the epithelium from the lamina propria. Fibroblasts can arise from Epithelial Mesenchymal transformation while also releasing and responding to cytokines.
Perspectives
The hallmark of tissue remodeling is inflammation. Therefore, this concept that fibroblast can function as a non-professional immune cell is really aligned with the receptor expression and reported functions. Leukocytes must be recruited to sites of inflammation for appropriate nondelayed healing [27,28]. If leukocytes (PMN, which are the first line of defense) are not, the inflammatory process is compromised [28]. Thus, fibroblast play a significant role in inflammation. Taken together the complete role of the fibroblast needs an expansion and a re-evaluation.
References
- Halper J, M Kjaer (2014) Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol 802: 31-47.
- Halper J (2018) Basic Components of Vascular Connective Tissue and Extracellular Matrix. Adv Pharmacol 81: 95-127.
- James J Tomasek, Giulio Gabbiani, Boris Hinz, Christine Chaponnier, Robert A Brown (2002) Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat Rev Mol Cell Biol 3(5): 349-363.
- Lamouille S, J Xu, R Derynck (2014) Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 15(3): 178-196.
- Duanqing Pei, Xiaodong Shu, Ama Gassama Diagne, Jean Paul Thiery (2019) Mesenchymal-epithelial transition in development and reprogramming. Nat Cell Biol 21(1): 44-53.
- Ian A Darby, Tim D Hewitson (2007) Fibroblast differentiation in wound healing and fibrosis. Int Rev Cytol 257: 143-179.
- Andrew A Gibb, Michael P Lazaropoulos, John W Elrod (2020) Myofibroblasts and Fibrosis: Mitochondrial and Metabolic Control of Cellular Differentiation. Circ Res 127(3): 427-447.
- Hinz B (2016) Myofibroblasts. Exp Eye Res 142: 56-70.
- Wynn TA, T R Ramalingam (2012) Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med 18(7): 1028-1040.
- Bellini A, S Mattoli (2007) The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab Invest 87(9): 858-870.
- Bucala R (2015) Fibrocytes at 20 Years. Mol Med 21 Suppl 1: S3-S5.
- Marta Ramirez, Nuria Pell, Marc Mejias, Mercedes Fernandez (2019) Pericytes in the Gut. Adv Exp Med Biol 1122: 73-100.
- Rita N Gomes, Filipa Manuel, Diana S Nascimento (2021) The bright side of fibroblasts: molecular signature and regenerative cues in major organs. NPJ Regen Med 6(1): 43.
- Karen E Porter, Neil A Turner (2009) Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther 123(2): 255-278.
- Sara Panizo, Laura Martínez Arias, Cristina Alonso Montes, Pablo Cannata, Beatriz Martín-Carro (2021) Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int J Mol Sci 22(1): 408.
- Cayce E Dorrier, Hannah E Jones, Lucija Pintarić, Julie A Siegenthaler, Richard Daneman (2022) Emerging roles for CNS fibroblasts in health, injury and disease. Nat Rev Neurosci 23(1): 23-34.
- Joo-Hyeon Lee, Tuomas Tammela, Matan Hofree, Jinwook Choi, Nemanja Despot Marjanovic (2017) Anatomically and Functionally Distinct Lung Mesenchymal Populations Marked by Lgr5 and Lgr6. Cell 170(6): 1149-1163.
- Alessandra Micera, Bijorn Omar Balzamino, Antonio Di Zazzo, Filippo Biamonte, Gigliola Sica (2016) Toll-Like Receptors and Tissue Remodeling: The Pro/Cons Recent Findings. J Cell Physiol 231(3): 531-544.
- Elżbieta Kimak, Kamil Nurczyk, Tomasz Skoczylas, Dariusz Duma, Renata Gieroba (2019) Fibroblast growth factor 21, epidermal growth factor receptor, interleukin 6, myeloperoxidase, lipid hydroperoxide, apolipoproteins A-I and B, as well as lipid and lipoprotein ratios as diagnostic serum biomarkers for gastric cancer. Pol Arch Intern Med 129(7-8): 559-562.
- Francesca Wanda Rossi, Filomena Napolitano, Ada Pesapane, Massimo Mascolo, Stefania Staibano, et al. (2015) Upregulation of the N-formyl Peptide receptors in scleroderma fibroblasts fosters the switch to myofibroblasts. J Immunol 194(11): 5161-5173.
- Giovanna Leoni, Jeannie Gripentrog, Connie Lord, Marcia Riesselman, Ronen Sumagin, et al. (2015) Human neutrophil formyl peptide receptor phosphorylation and the mucosal inflammatory response. J Leukoc Biol 97(1): 87-101.
- Jun Liu, Yan Luo, Liming Zheng, Qingqing Liu, Zhongcai Yang, et al. (2013) Establishment and characterization of fetal fibroblast cell lines for generating human lysozyme transgenic goats by somatic cell nuclear transfer. Transgenic Res 22(5): 893-903.
- Iuliano R (1991) [Lactoferrin]. Minerva Med 82(12): 799-805.
- Takayama Y, T Takezawa (2006) Lactoferrin promotes collagen gel contractile activity of fibroblasts mediated by lipoprotein receptors. Biochem Cell Biol 84(3): 268-274.
- Roulis M, RA Flavell (2016) Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 92(3): 116-131.
- Chang-Youh Tsai, Song-Chou Hsieh, Chih-Wei Liu, Cheng-Shiun Lu, Cheng-Han Wu, et al. (2021) Cross-Talk among Polymorphonuclear Neutrophils, Immune, and Non-Immune Cells via Released Cytokines, Granule Proteins, Microvesicles, and Neutrophil Extracellular Trap Formation: A Novel Concept of Biology and Pathobiology for Neutrophils. Int J Mol Sci 22(6): 3119.
- Robert Pick, Daniela Begandt, Thomas J Stocker, Melanie Salvermoser, Sarah Thome, et al. (2017) Coronin 1A, a novel player in integrin biology, controls neutrophil trafficking in innate immunity. Blood 130(7): 847-858.



 We use cookies to ensure you get the best experience on our website.
We use cookies to ensure you get the best experience on our website.